Nature's Gift: New Paper Reveals Advances in Cannabidiol CBD Antitumor Mechanisms
Chinese medicine ghostwriter Ni Haixia once said that marijuana is the best Chinese medicine. Because marijuana is a plant, pharmaceutical companies can't go for a patent because the patent belongs to God ......
A recent review paper published on the National Library of Medicine under the U.S. Department of Health and Human Services (HHS) is as follows:
Cannabidiol (CBD), a non-psychoactive ingredient derived from the Cannabis plant, has shown therapeutic effects in a variety of disorders, including anxiety, neurological disorders, inflammation, and tumors.CBD exerts its antitumor effects by regulating the cell cycle, inducing apoptosis and autophagy in tumor cells, and inhibiting tumor cell invasion, migration, and angiogenesis. This article reviews the anti-tumor mechanism of CBD, aiming to provide reference for clinical treatment of tumor diseases and rational use of CBD.
Literature Link: https://pubmed.ncbi.nlm.nih.gov/38731434/
1、Introduction to the Cannabinoid CBD
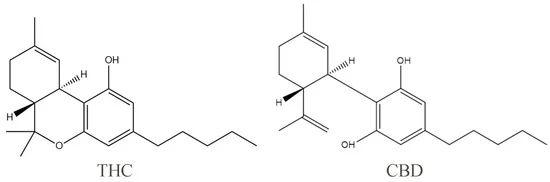
Cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC) are pure natural compounds extracted from the cannabis plant and are the two most widely studied phytocannabinoids. One of the properties of pure CBD is that it has light yellow crystals that are readily soluble in organic solvents such as ethanol, methanol, and ether, and virtually insoluble in water. The phenolic hydroxyl group contributes to the highest occupied molecular orbital value of CBD, and thus the phenolic hydroxyl group is the structural basis for the antioxidant properties of cannabidiol.
With further research, CBD has also been found to have anti-inflammatory, anti-tumor, and antiemetic properties. THC is the main psychoactive component of cannabis, which acts as a partial agonist, binding to the appropriate receptors and exerting a variety of physiological effects. Studies have shown that THC has significant analgesic and antiemetic effects, but its clinical use is limited by psychiatric side effects such as anxiety and cognitive impairment. Unlike THC, CBD does not impair the psychiatric system and, in fact, prevents and reverses anxiety and addiction caused by THC-induced over-activation of the extracellular regulated kinase (ERK) pathway.
The antitumor activity of CBD has attracted increasing attention and has been demonstrated in a variety of tumor types such as colon, lung, breast, ovarian, and prostate cancer.The antitumor mechanisms of CBD include induction of cell cycle arrest and autophagy, promotion of apoptosis, regulation of angiogenesis, and inhibition of tumor cell migration and invasion.
In addition, CBD has good synergistic effects with other drugs, and some clinical reports have shown that CBD is used in the treatment of cancer. In this paper, we present a review on the mechanism of CBD inhibition of tumor growth and metastasis, the synergistic effect of CBD with other antitumor drugs, and the research progress of CBD reversal of tumor resistance. The overall aim is to provide an important reference for the clinical application of CBD therapy.
2、Anti-tumor mechanism of CBD
Unlike normal cells, tumor cells have the characteristics of infinite replication, anti-apoptosis, continuous angiogenesis, tissue invasion and metastasis.CBD, as a potential tumor therapeutic drug, achieves its anti-tumor effects by regulating multiple processes that govern tumorigenesis and development.
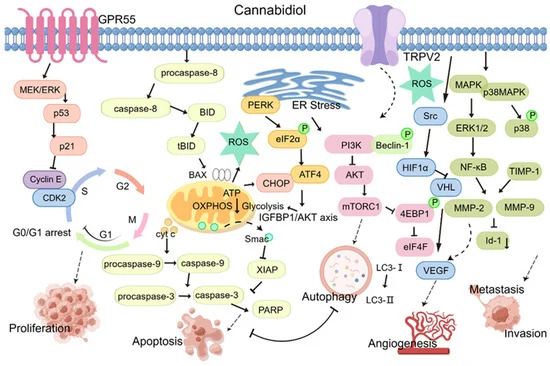
Figure 2.The anti-tumor activity of CBD (proliferation, apoptosis, autophagy, angiogenesis, invasion and metastasis).
2.1. CBD Regulates Tumor Cell Cycle
Cell proliferation is regulated by the cell cycle, and blocking the division cycle of tumor cells is an important anti-tumor mechanism. Eukaryotic cells rapidly synthesize RNA and proteins through the G1 phase, which provides material and energy for DNA synthesis in the S phase.The G2 phase assembles the materials necessary for cell division and prepares cells for the M phase or mitosis. Therefore, in addition to overcoming the S and M phases to inhibit tumor cell proliferation, regulating the G1 and G2 phases is important for tumor therapy. Several studies have shown that CBD treatment can sensitize tumor cells to G0-G1 phase blockade.
In thyroid and breast cancer cells, CBD exerts its antiproliferative effect by causing cell cycle arrest during the G1/S phase transition, but does not have a similar effect in non-tumor cells. In pancreatic cancer, CBD inhibits cell cycle progression by binding to the GPR55 receptor and dose-dependently blocking its activity, resulting in down-regulation of the MAPK/ERK signaling pathway, inhibition of the G1/S transition, reduction of S-phase DNA synthesis, and inhibition of pancreatic cancer cell growth.
The cell cycle is regulated by cell cycle proteins and cell cycle protein-dependent kinases (CDK). Cell cycle proteins and CDKs can bind to form heterodimers that promote cell cycle progression. cyclinE forms a complex with CDK2 that promotes the G1/S transition.
Zhang et al. showed that CBD exerts its antiproliferative effect by significantly downregulating the expression of p53 protein, upregulating the expression of p21 protein and ataxia telangiectasia mutated genes, and inhibiting the expression of gastric cancer cell cycle proteins E and CDK2, resulting in cell cycle arrest in the G0-G1 phase. CBD exerts its anti-glioblastoma effect by specifically regulating the activity of ERK and various caspase-3, caspase-7 and caspase-9, resulting in the arrest of U251 and SF 126 cells in the G0-G1 phase. Combination therapy consisting of CBD and THC significantly enhanced this inhibitory effect.
2.2. Multiple CBD-related pathways induce apoptosis in tumor cells Apoptosis is the autonomous and orderly death of cells.
It is genetically controlled and is associated with the process of actively eliminating redundant or differentiated cells that are incompatible with the body, cells that have fulfilled their function but are no longer used/needed, and potentially dangerous cells. Thus, apoptosis is considered an indispensable regulatory mechanism for the normal development of organisms; however, when out of control, apoptosis can lead to a variety of diseases, including tumorigenesis. Defective or impaired apoptosis can disrupt the normal proliferation, differentiation and death programs of normal cells, and this disruption is one of the potential factors in tumorigenesis.
Currently, clinically used radiotherapy and chemotherapy drugs are mainly used to induce apoptosis in tumor cells and show efficacy. Recent findings have confirmed that CBD can exert its antitumor effects by inducing apoptosis in tumor cells through several processes, including disruption of mitochondrial homeostasis and implementation of endoplasmic reticulum stress (ERS).
Mitochondria are the active center of apoptosis regulation and play an important role in the apoptotic pathway. Under normal physiological conditions, various antioxidant systems in cells and mitochondria regulate mitochondrial reactive oxygen species (ROS) production to maintain intracellular ROS homeostasis. Perturbation of mitochondrial ROS homeostasis is an important factor in the development of disease in vivo.
In breast cancer MDA-MB-231 cells, CBD induced the production and translocation of BH3-interacting structural domain death agonist (BID), increased cytochrome C release, and ultimately promoted apoptosis by decreasing mitochondrial membrane potential. In addition, CBD treatment increases ROS production. Limiting ROS levels with the antioxidant α-tocopherol (TOC) reduces CBD-induced expression of apoptotic proteins, again suggesting that ROS are involved in apoptosis.
Other studies have shown that CBD activates the cysteinyl asparaginase cascade reaction in leukemic Jurkat cells, leading to PARP cleavage, up-regulation of NADH oxidase expression, and an increase in ROS production, which ultimately leads to apoptosis.Massi et al [17] reported that CBD activates cysteinyl asparaginase-8 and cysteinyl asparaginase-9 through early ROS production and glutathione (GSH) depletion to induce apoptosis in glioma cells.
Another apoptosis trigger is ERS, which is defined as the accumulation of unfolded or misfolded proteins in the endoplasmic reticulum in response to hypoxia, acidosis, and other stress stimuli. Apoptosis can be induced by high-intensity and prolonged stress responses. In colon cancer cells, CBD induced ERS and increased the expression of the death receptor DR5 by phosphorylating double-stranded RNA-dependent protein kinase-like ER kinase-C/EBP homologous protein (PERK-CHOP) and also enhanced tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in tumor cells.
In addition, CBD dose-dependently induced apoptosis in gastric cancer cell lines (AGS, MKN45, SNU638, and NCI-N87).The molecular mechanism of action of CBD involves induction of ERS and mitochondrial dysfunction by translocation of the pro-apoptotic factor Smac to the cytoplasm to bind to the X-linked inhibitor of apoptosis protein (XIAP), which increases the ubiquitin expression of XIAP in gastric cancer cells. ubiquitination of XIAP in gastric cancer cells.
Sreevalsan et al. showed that CBD promotes phosphatase-dependent and/or CB receptor-dependent apoptosis in prostate cancer LNCaP and colon cancer SW 480 cells. In lung cancer, CBD induced apoptosis in H460 and A450 cells through upregulation of the adhesion molecule ICAM-1 and lymphokine-activated killer (LAK)-mediated tumor cell death by CBD.
MicroRNAs (miRNAs) are short non-coding RNAs that may also be involved in tumor pathogenesis by regulating apoptosis. Alharis et al. published the first report showing that CBD can control tumor cell invasion, migration and apoptosis through miRNAs.CBD specifically downregulates miRNA hsa-let-7a and upregulates the expression of caspase-3 and GAS-7 genes. These changes, in turn, activate neuroblastoma (NBL) cell apoptosis.
2.3. CBD induces autophagy of tumor cells
In addition to its pro-apoptotic effects, CBD has shown the ability to induce autophagy in tumor cells. Autophagy is considered a type II programmed cell death, which includes cell death triggered by the degradation of misfolded proteins or dysfunctional organelles. Autophagy plays a key role in all stages of tumorigenesis, development, metabolism and metastasis and thus has a dual impact on many malignancies.
In the early stages of tumorigenesis, autophagy potentially hinders carcinogenesis by eliminating defective proteins and organelles. Unfortunately, in advanced stages of cancer progression, especially when tumors are exposed to stressors (e.g., angiogenesis restriction, nutrient deprivation, and hypoxia), autophagy assumes a pro-survival role and instead promotes tumor proliferation, metastasis, and invasion. Therefore, cellular autophagy has become a focus of tumor therapy. Therefore, the exact mechanism by which CBD exerts its anti-tumor effects by stimulating autophagy in tumor cells has attracted much attention.
CBD induced the aggregation of LC 3-II protein, an autophagy marker, in human colon epithelial cell lines. During autophagy, LC 3 protein is synthesized and hydrolyzed by ATG 4 to produce cytoplasmic LC 3-I, which then covalently binds to phosphatidylethanolamine in the presence of Atg 7 and Atg 3. LC 3-I is subsequently lipoylated to form LC 3-II, which binds to the autophagosome membrane to form the autophagosome [29]. This process has been demonstrated in several colorectal cancer (CRC) cell lines.
Furthermore, CBD-induced LC3-II expression was observed in glioma cells. However, in these cells, CBD enhanced early autophagy flux rather than promoting the complete autophagy process.CBD-induced cell death could be blocked by early autophagy inhibitors (wortmannin and LY294002), whereas late autophagy inhibitors (CQ and Baf-A1) promoted cell death.
The autophagic process is closely related to apoptosis, and several studies have confirmed that CBD-induced autophagic responses sometimes coexist with apoptosis. One of these responses is associated with Beclin-1, a mammalian autophagy gene and a key target in the regulation of autophagy.Beclin-1 can bind to Bcl-2 family proteins to form protein complexes that regulate autophagy levels.CBD can inhibit the linkage between Beclin1 and Bcl-2, which can increase autophagy levels.
In the context of breast cancer MDA-MB-231 cells, CBD treatment has been observed to induce ERS, leading to the accumulation of LC3-II and subsequent triggering of autophagy-mediated cell death. In this case, the process of apoptosis produces a significant amount of caspase-3, which in turn cleaves Beclin-1, thereby impeding autophagy. Concurrently, the cleavage product undergoes mitochondrial translocation, inducing apoptosis by facilitating Cytochrome C release.
The predominance of apoptosis and autophagy appeared to be determined by the concentration of CBD.CBD also up-regulated the acute myeloid leukemia (AML)-1 transcription factor, bound the transient receptor potential vanilloid-2 (TRPV 2) promoter in a PI 3 K/AKT-dependent manner, and activated TRPV 2 to trigger the differentiation of glioma stem cells (GSCs), which activated autophagic processes and inhibited GSC growth.
In addition, ex vivo experiments have demonstrated that the combination treatment of CRC cells with CBD and oxaliplatin reduces nitric oxide synthase 3 (NOS 3) activity, leading to mitochondrial dysfunction-mediated excessive ROS production and endoplasmic reticulum (ER) stress, which ultimately induces autophagy. Consequently, this combination treatment exerts antitumor effects.
2.4. CBD has been demonstrated to impede the process of angiogenesis in tumor cells.
Angiogenesis is a dynamic process involving endothelial cell proliferation, adhesion, migration, and vascular ring formation. This process is regulated by angiogenic mediators and inhibitors. Uncontrolled angiogenesis can lead to various diseases, including several immune disorders (rheumatoid arthritis), tumorigenesis, and tumor metastasis. Numerous studies support the hypothesis that CBD can exert its antitumor effects by inhibiting tumor angiogenesis.
For instance, Solinas et al. found that CBD significantly interfered with and decreased the protein expression of several regulatory factors involved in the angiogenic process in glioma U87-MG cells, such as vascular endothelial growth factor (VEGF), matrix metalloproteinase-2/9 (MMP-2/9), matrix metalloproteinase inhibitor-1 (TIMP-1), urokinase-type fibrinogen activator (uPA), etc. However, no specific signaling pathways were identified.
The matrix metalloproteinase family (MMPs) has been demonstrated to play a role in the process of angiogenesis through the processes of basement membrane disassembly and extracellular matrix remodeling. Conversely, TIMP-1, an endogenous metalloproteinase inhibitor, has been shown to impede the activity of MMPs.HIF-1α, a pivotal regulator of oxygen homeostasis, has been identified as a critical factor in tumor angiogenesis and metastasis.
A recent study demonstrated that cannabidiol (CBD) can impede breast cancer proliferation by suppressing SRC activity, which upregulates von Hippel-Lindau (VHL) expression, reduces hypoxia-inducible factor-1α (HIF-1α) synthesis in breast cancer cells, and inhibits angiogenesis. Tumor-associated macrophages secrete EGF and promote angiogenesis. In a study on the role of CBD in breast cancer, it was reported that CBD inhibits angiogenesis and thus exerts anti-tumor effects. It modulates the tumor microenvironment by regulating cytokines produced by tumor cells, thereby reducing the recruitment of total and M2 macrophages to primary and secondary tumor sites.
Despite the extensive research conducted on the role of cannabinoids and their analogs in the inhibition of angiogenesis, the antiangiogenic effects of CBD remain to be fully elucidated. In addition to the established targets of CBD's antiangiogenic effects, a multitude of small molecules and proteins have been identified as potential inhibitors of angiogenesis, warranting further exploration.
For instance, endothelial TRPV4 has been demonstrated in recent years to be a pivotal regulator of vascular integrity and tumor angiogenesis, implying that targeting TRPV4 could emerge as a novel strategy for vascular normalization and cancer therapy. Given the established role of CBD in activating TRPV4, it is conceivable that CBD's anti-angiogenic effects may be, at least in part, attributable to its ability to target TRPV4. Furthermore, GPR55, a potential receptor for CBD, has been identified as a regulator of endothelial cell proliferation, with the capacity to reduce nerve growth factor (NGF) levels in the tumor microenvironment (TME). Consequently, further exploration into the intricate interplay between these receptors is warranted, as it may unveil novel strategies for hindering tumor-induced angiogenesis.
2.5. CBD inhibits tumor cell invasion and migration
The hallmark of malignant neoplasms is the capacity of their cells to invade and migrate over considerable distances. These metastatic cells are also significant contributors to mortality in clinical cancer patients. The findings of the present study corroborate the capacity of CBD to impede the invasion and migration of diverse tumor cell types.
Epithelial mesenchymal transition (EMT) has garnered significant attention due to its potential role in the transformation of benign tumors to invasive and metastatic malignancies.EMT is a pivotal process in tumor invasion and metastasis. During the EMT process, numerous genes undergo altered expression, including those associated with an epithelial phenotype (e.g., E-calmodulin, keratins, and tight junction protein-1) and those associated with a mesenchymal phenotype (e.g., N-calmodulin, waveform proteins, and fibronectin). Thus, targeting EMT-associated signals is a promising strategy to halt tumor progression.
In CRC cells treated with CBD, the EMT process was found to be inhibited, which was attributed to the disruption of the Wnt/β-collagen signaling pathway. This intervention led to an increase in the expression of E-calmodulin and a decrease in the expression of N-calmodulin. These effects effectively blocked the Wnt pathway and prevented cancer cell migration.
In a similar manner, in breast cancer cells, CBD exhibited the capacity to restore epithelial organization that had been lost due to cell dispersion by promoting the relocalization of E-calmodulin and β-catenin at cell adhesion junctions.Furthermore, CBD restored epithelial organization by blocking IL-1β/IL-1-induced IL-1β/IL-1 RI/β-catenin signaling pathway to prevent nuclear translocation of β-catenin, which regulates migration and progression, one of the key factors driving EMT.
Consequently, CBD has the potential to impede tumor invasion and migration by reversing EMT. Another target of CBD with the potential to inhibit invasion is the fibrinogen activation system. In this system, fibrinogen activator inhibitor 1, a serine protease inhibitor, functions by impeding fibrinogen activators and hindering the invasion of malignant tumor cells. In addition, evidence suggests that CBD can inhibit cell invasion by suppressing lung cancer A549 cell expression through a cannabinoid receptor 1 (CB1)/cannabinoid receptor 2 (CB2)/TRPV 1-dependent pathway.
Matrix metalloproteinases (MMPs), synthesized and secreted by tumor cells, target multiple proteins in the extracellular matrix (ECM) and degrade the ECM, leading to tissue structural relaxation and promoting tumor cell invasion and metastasis.CBD has been shown to impede the activation of the EGF/EGFR signaling pathway and the activity of its downstream targets, AKT, ERK, and NF-κB, thereby hindering the proliferation of tumor cells. Additionally, CBD has been observed to curtail cell invasion by downregulating the secretion of MMP-2 and MMP-9, thus impeding the invasion and migration of breast cancer cells.
The study identified the transcription regulator Id-1 as a crucial factor in the development of various types of cancer, playing a pivotal role in tumorigenesis and metastasis. In breast cancer cells, McAllister et al. found that the compound with the most significant inhibitory effect on tumor cell proliferation, invasion, and migration was CBD. Further study of the related mechanism of action revealed that CBD inhibited the expression of the transcriptional regulator Id-1 in breast cancer cells by simultaneously increasing p-ERK and ROS, thereby decreasing cellular expression of the transcriptional regulator Id-1. The study's findings suggest that CBD's ability to inhibit Id-1 expression in breast cancer cells may be a promising avenue for further research, with implications for the development of novel therapeutic strategies.
Furthermore, CBD elicited analogous effects in glioma cells, exhibiting a substantial decrease in expression.
A seminal study utilizing a pre-established murine model of breast cancer metastasis corroborated the finding that CBD curtailed the development of total breast cancer metastases to the lungs by up to 75%, impeded the proliferation of lung metastases, and exhibited superior efficacy in its capacity to impede tumor invasion compared to its efficacy in repressing the growth of primary tumors.
2.6. CBD exerts its effects on the energy metabolism of tumor cells.
A distinguishing characteristic of tumors is the metabolic reprogramming of tumor cells. Abnormal metabolism within tumor cells has been shown to accelerate malignant progression. The predominant metabolic pathways encompass glycolysis, oxidative phosphorylation, amino acid metabolism, and fatty acid metabolism, among others. Under normal conditions, aerobic oxidation serves as the primary energy pathway within the body. However, under conditions of hypoxia, the body shifts to glycolysis as its predominant energy production pathway. Wahlberg noted that even under aerobic conditions, tumor cells are more likely to be powered by glycolysis.
Notably, glycolysis does generate ATP, which is essential for the vital activities of tumor cells. Moreover, it supplies intermediates that are crucial for protein and lipid synthesis. This process contributes to the growth and proliferation of tumor cells. This observation suggests the potential for CBD to elicit anti-tumor effects by modulating the energy metabolism of tumor cells.
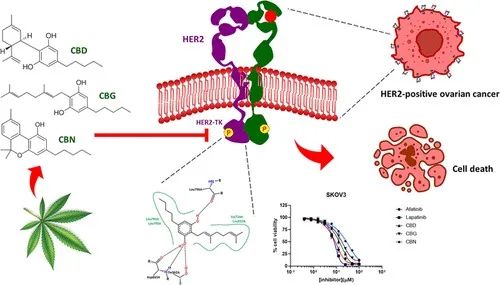
Studies have demonstrated that CBD significantly reduces the basal cellular respiration rate and total ATP production, thereby hindering the growth of human gastric cancer AGS cells. A similar effect was observed in neuroblastoma (NBL) cells.CBD retarded the growth of NBL cells by inhibiting mitochondrial respiration, although the rate of glycolysis was not significantly altered. To date, however, there is a paucity of reports addressing the specific mechanisms by which CBD regulates bioenergetic metabolism in tumor cells. Our recent study suggests that CBD can inhibit ovarian cancer cell growth by disrupting LAIR-1-mediated mitochondrial bioenergetic metabolism and the PI 3 K/AKT/mTOR pathway.
As demonstrated by Shangguan et al., cannabidiol (CBD) has the capacity to impede the proliferation of hepatocellular carcinoma (HCC) cells, specifically HepG 2 and MHCC 97 H, by modulating the ATF 4-IGFBP 1-AKT axis and reducing the overall rate of aerobic glycolysis and energy metabolism. Sun et al. employed single-cell RNA sequencing (scRNA-seq) and single-cell ATAC sequencing (scATAC-seq) methods to examine the tumor microenvironment (TME) of MC38 xenograft tumors in mice after CBD administration.
Their findings revealed that CBD inhibited oxidative phosphorylation and fatty acid oxidation in macrophages by impeding the PI 3 K-Akt signaling pathway and its associated downstream target genes, thereby promoting glycolysis. Furthermore, CBD led to a substantial decrease in alternatively activated M2 macrophages and an increase in M1 macrophages, which significantly promoted anti-tumor immunity and hindered tumor growth in hormonal mice.
Tumor drug resistance remains a significant challenge in achieving successful tumor therapy. Metabolic reprogramming, particularly of the glycolytic pathway, has been identified as a key factor in drug resistance in malignant tumors. A recent study in a transgenic mouse model of prostate cancer confirmed that CBD inhibits the development of refractory prostate cancer (HRPC) in this model by reprogramming metabolic and oncogenic-related signals.
Mechanistically, CBD inhibited oxidative phosphorylation in enzalutamide-resistant HRPC cells by increasing glycolytic capacity and inhibiting oxidative phosphorylation.This effect of CBD stems from its ability to inhibit the development of prostate cancer by regulating the PI3K/Akt/mTOR pathway by inhibiting mitochondrial voltage-dependent anion channel 1 (VDAC1), a key protein in the mitochondrial outer membrane involved in the control of mitochondrial and cellular functions.
The regulation of mitochondrial function and apoptosis is coupled to hexokinase II (HK-II) activity. The interruption of the ATP supply required for the HK-II response results in apoptosis and autophagy. Consequently, when the VDAC 1 oligomerization inhibitor DIDS was administered in conjunction with CBD, the effects of CBD on cell survival and mitochondrial ATP production were significantly diminished. This finding underscores the potential for modifying tumor cell resistance in response to changes in endogenous metabolism, a concept that may inform the development of novel therapeutic agents aimed at overcoming drug resistance.
3. Synergistic sensitization and drug resistance reversal of antitumor drugs
Chemotherapy is a prevalent clinical treatment for various forms of cancer; however, patients often exhibit chemoresistance, which can result in treatment failure. The active constituents of numerous herbal products have been shown to reverse chemotherapy resistance in tumor cells. There is evidence that CBD can be used as an antitumor drug sensitizer in the treatment of malignant tumors and that CBD can effectively reverse tumor resistance. A substantial body of preclinical research, encompassing both cellular and animal experiments, has investigated the potential of CBD when utilized in conjunction with antitumor medications. The following section will outline the various CBD combinations that have been assessed in different tumor types.
CBD has been observed to exhibit a synergistic effect when used in conjunction with chemotherapeutic agents.The use of DNA-damaging drugs remains a prevalent approach in the management of certain malignancies, yet resistance to these agents has been documented. Some studies have shown that CBD can increase calcium uptake through TRPV2 channels to increase calcium ion uptake, thereby enhancing cellular uptake of chemotherapeutic agents and subsequently increasing the sensitivity of glioma cells to chemotherapeutic agents, including DNA-damaging drugs (temozolomide, carmustine, or cisplatin).
Furthermore, Massi et al. demonstrated in ex vivo experiments using glioma tissues and cells that CBD inhibits tumor growth by modulating the lipoxygenase (LOX) pathway and the endogenous cannabinoid system. Pretreatment with the 5-LOX-specific inhibitor MK-886 enhanced the anti-mitotic capacity of CBD, suggesting that CBD and 5-LOX inhibitors enhance the action of CBD. However, TRAIL acts simultaneously with the corresponding receptors, but also leads to resistance and short plasma half-life.In their study, Kim J.L. et al. found a synergistic antitumor effect of cannabidiol (CBD) with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in vitro, which could increase its sensitivity. However, this was not observed in normal colon cells.
A recent study demonstrated that the combination of cannabidiol (CBD) and photodynamic therapy (PDT) enhances the production of cytotoxic substances, stimulates the patient's immune system, and interferes with multiple signaling pathways associated with the regulation of tumorigenesis, drug resistance, and metastasis. This results in the prevention of secondary spread of colorectal cancer stem cells and the enhancement of the therapeutic efficacy of PDT in colorectal cancer. Mokoena et al. also noted a strong in vitro killing effect of the combination of CBD and PDT on MCF-7 breast cancer cells, and they proposed that the mechanism may be related to the induction of apoptosis. In the context of hepatocellular carcinoma, cannabidiol has been observed to promote cabozantinib-induced tumor cell death by modulating p53 phosphorylation under ERS.
Furthermore, several studies have demonstrated that GSC are significantly resistant to treatment with the conventional antitumor drug carmustine (also known as BCNU), as the cell viability of GSC decreases only slightly after BCNU treatment. However, when CBD is administered in conjunction with BCNU, the high resistance of GSC to BCNU treatment is overcome by an enhancement of BCNU-induced apoptosis, a process that is dependent on CBD.
The role of reactive oxygen species (ROS) in glioblastoma resistance has also been examined, and CBD treatment of mice bearing GSC tumors has been shown to significantly increase their tumor ROS levels. This led to the inhibition of the survival and self-renewal of GSC tumor cells and a significant increase in the survival rate of the mice.The combination of CBD with antioxidant-responsive gene inhibitors further enhanced the ability to inhibit tumor growth, invasion, and GSC self-renewal.
Jeong et al. [34] established oxaliplatin-resistant DLD-1R and Colo 205 R colorectal cancer cell lines and demonstrated that elevated levels of NOS 3 phosphorylation were critical for oxaliplatin resistance. Furthermore, they demonstrated that the combination of cannabidiol (CBD) and oxaliplatin induced reactive oxygen species (ROS) overproduction through mitochondrial dysfunction, which further inhibited NOS 3 phosphorylation, thereby overcoming oxaliplatin resistance.
In a similar manner, the combination of cannabidiol (CBD) and imatinib, a targeted drug used to treat chronic granulocytic leukemia (CML), reversed imatinib resistance.Ozmen et al. demonstrated by pathohistological examination that CBD treatment reversed the pathology of congestion, edema, inflammatory cell infiltration, and epithelial cell loss in the lungs that is usually associated with the use of methotrexate (MTX). MTX is a drug frequently used in the treatment of various cancers, but its use can have adverse effects on organs, especially the lungs.
Extracellular vesicles (EVs) include exosome and apoptosis vesicles (EMVs), which play an important role in the physiological and pathological processes of the organism. The release of EMV in tumors has been associated with chemotherapy resistance in cancer cells.
In a study by Kosgodage et al., it was demonstrated that CBD significantly reduced the release of both EXOs and EMVs in three distinct cancer cell lines, including prostate cancer (PC3), hepatocellular carcinoma (HEPG2), and breast cancer (MDA-MB-231). Furthermore, the modulatory effects of CBD were found to be dose-dependent and tumor cell type-specific, resulting in an increased sensitivity of tumor cells to chemotherapy.
In recent years, the field of drug-carrying particle technology has seen significant progress in the delivery of antitumor drug combinations for precise targeting, reduced risk of metastasis, and reduced tumor resistance. A team of researchers evaluated the synergistic activity of cannabidiol (CBD) in combination with paclitaxel (PTX) for the treatment of ovarian cancer and found that the dosage of PTX could be reduced, thereby decreasing drug-related toxicity and drug resistance. Furthermore, PLGA particles have been utilized as a carrier for CBD, thereby enhancing the antitumor activity of PTX in comparison to Adriamycin and cisplatin.
Indeed, CBD exhibited antagonistic effects. Another study demonstrated that the incorporation of CBD into a bionic carbon monoxide nanocomplex (HMPOC@M) augmented autophagy flux and promoted cancer cell death through the activation of class III phosphatidylinositol 3-kinase (PI 3 K-III)/Beclin-1 complex. In vivo experiments demonstrated that HMPOC@M+ laser treatment strongly attenuated liver and lung metastases and inhibited tumor growth by down-regulating VEGF and MMP 9 proteins.
4. Clinical applications of tumor control
Although a considerable body of research has investigated the anti-tumor mechanisms of CBD in various cancer types through in vitro and preclinical in vivo studies, there is a paucity of clinical trials that have employed CBD as a cancer treatment.Kenyon J. et al. systematically analyzed data from 119 cancer patients, reporting that approximately 92% of patients with solid tumors demonstrated clinical improvement following treatment with synthetic CBD drugs. The improvements observed included tumor shrinkage and a reduction in the number of circulating cells, with no significant adverse effects documented.
In a separate report, Barry et al. documented the case of a patient diagnosed with plasma ovarian cancer who underwent a two-month treatment regimen involving nightly sublingual drops of Cbd Oil, administered in conjunction with bitter almond tablets containing mandelic acid. The patient's subsequent CT scans revealed a bilateral reduction in tumor size and an increase in the size of the pelvic lymph nodes. Concurrently, Sule-Suso et al. documented the case of an elderly patient with lung adenocarcinoma who underwent one-month CBD oil treatment, resulting in a nearly complete disappearance of the mass in the left lower lobe and a substantial reduction in mediastinal lymph nodes, as evidenced by CT scans. These clinical cases, which pertain to different types of cancer, provide compelling evidence that CBD use has significant benefits for oncology patients.However, it is important to note that further refinement of clinical trial studies of CBD is necessary to ensure the most accurate and reliable results.
5. Summary and Prospects
The anti-tumor effects of CBD in different types of cancers have attracted widespread attention, and the number of related research results is steadily increasing. The extant studies have indicated that CBD exerts substantial antitumor effects, with mechanisms that encompass cell cycle arrest and autophagy induction, promotion of apoptosis, regulation of angiogenesis, and inhibition of tumor cell migration and invasion. Furthermore, CBD has demonstrated a notable synergistic effect when used in conjunction with other pharmaceutical agents, as evidenced by clinical reports highlighting its use in cancer treatment. The findings of this review indicate that CBD has considerable potential for clinical utilization in the management of cancer patients.
However, it is crucial to note that CBD's potential benefits are currently hindered by its structural limitations, which include a simple structure, poor aqueous solubility, and low bioavailability. These limitations, in turn, result in significant inhibition of its antioxidant properties and stability, thereby limiting its clinical application. Polymer synthesis, structural modification, and the introduction of hydrophilic groups have been demonstrated to enhance the antioxidant properties of CBD and improve its solubility and permeability.
Despite the paucity of related studies, this provides a foundation for further in vivo experimental studies and clinical applications of CBD, which shows great promise.The antitumor mechanism of CBD appears to be multifaceted. To this end, further detailed and in-depth studies are needed to provide a stronger theoretical basis for advancing the clinical application of CBD.